Peeking into healthy brains to see if Alzheimer’s is brewing
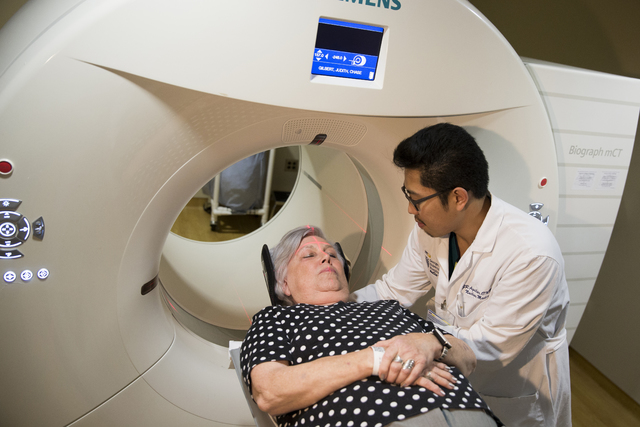
WASHINGTON — Sticky plaque gets the most attention, but now healthy seniors at risk of Alzheimer’s are letting scientists peek into their brains to see if another culprit is lurking.
ADVERTISING
No one knows what actually causes Alzheimer’s, but the suspects are its two hallmarks — the gunky amyloid in those brain plaques or tangles of a protein named tau that clog dying brain cells. New imaging can spot those tangles in living brains, providing a chance to finally better understand what triggers dementia.
Now researchers are adding tau brain scans to an ambitious study that’s testing if an experimental drug might help healthy but at-risk people stave off Alzheimer’s. Whether that medication works or not, it’s the first drug study where scientists can track how both of Alzheimer’s signature markers begin building up in older adults before memory ever slips.
“The combination of amyloid and tau is really the toxic duo,” predicted Dr. Reisa Sperling of Boston’s Brigham and Women’s Hospital and Harvard Medical School, who is leading the so-called A4 study. “To see it in life is really striking.”
The A4 study — it stands for Anti-Amyloid Treatment in Asymptomatic Alzheimer’s — aims to enroll 1,000 healthy seniors like Judith Chase Gilbert, 77, of Arlington, Virginia. The recently retired government worker is mentally sharp but learned through the study that her brain harbors amyloid buildup that might increase her risk. Last week, researchers slid Gilbert into a doughnut-shaped PET scanner as she became one of the first study participants to also have their brains scanned for tau.
“We know that tau starts entering the picture at some point, and we do not know when. We do not know how that interaction happens. We should know,” said chief science officer Maria Carrillo of the Alzheimer’s Association, which is pushing to add tau scans to other dementia research, too.
More than 35 million people worldwide have Alzheimer’s or similar dementias, including about 5 million in the U.S. Those numbers are expected to rise rapidly as the baby boomers get older. There is no good treatment. Today’s medications only temporarily ease symptoms and attempts at new drugs, mostly targeted at sticky amyloid, have failed in recent years.
Maybe that’s because treatment didn’t start early enough. Scientists now think Alzheimer’s begins quietly ravaging the brain more than a decade before symptoms appear, much like heart disease is triggered by gradual cholesterol buildup. Brain scans show many healthy older adults quietly harbor those sticky amyloid plaques, not a guarantee that they’ll eventually get Alzheimer’s but an increased risk.
Yet more recent research, including a large autopsy study from the Mayo Clinic, suggests that Alzheimer’s other bad actor — that tangle-forming tau protein — also plays a big role. The newest theory: Amyloid sparks a smoldering risk, but later spread of toxic tau speeds the brain destruction.
Normal tau acts sort of like railroad tracks to help nerve cells transport food and other molecules. But in Alzheimer’s, the protein’s strands collapse into tangles and eventually the cell dies. Most healthy people have a small amount of dysfunctional tau in one part of the brain by their 70s, Sperling said. But amyloid plaques somehow encourage this bad tau to spread toward the brain’s memory center, she explained.
The A4 study, which is enrolling participants in the U.S., Australia and Canada, may give some clues.
The goal is to check up to 500 people for tau three times over the three-year study, as researchers tease out when and how it forms in those who are still healthy. They won’t be told the results — scientists don’t know enough yet about what the scans portend.
At the same time, study participants will receive either an experimental anti-amyloid drug — Eli Lilly & Co.’s solanezumab — or a placebo as researchers track their memory. The $140 million study is funded by the National Institutes of Health, Lilly and others; the Alzheimer’s Association helped fund the addition of the tau scans.
The idea: If the drug proves to be helpful, it might be tamping down amyloid formation that in turn reins in toxic tau. In previous studies, solanezumab failed to help full-blown Alzheimer’s but appeared to slow mental decline in patients with mild disease, raising interest in testing the still healthy.
“We’re trying to remove amyloid’s downstream effects on tau formation,” said Dr. R. Scott Turner of Georgetown University Medical Center, where Gilbert enrolled in the study.
Seeing how amyloid and tau interact in living brains “is opening a whole new chapter into possible therapies,” Turner added.
For Gilbert, learning she had amyloid buildup “was distressing,” but it has prompted her to take extra steps, in addition to the study, to protect her brain. On her doctor’s advice, she’s exercising more, and exercising her brain in a new way by buying a keyboard to start piano lessons.
“It’s exciting to be part of something that’s cutting edge,” said Gilbert, who had never heard of tau before.
And she has a spot-on question: “So what’s the medication for the tau?”
Stay tuned: A handful of drugs to target tau also are in development but testing will take several years.


