WASHINGTON — Pfizer said Wednesday that it will supply the U.S. government with an additional 100 million doses of its COVID-19 vaccine under a new agreement between the pharmaceutical giant and the Trump administration.
Pfizer and its German partner BioNTech said that will bring their total current commitment to 200 million doses for the U.S. That should be enough to vaccinate 100 million people with the two-shot regimen. The government also has an option to purchase an additional 400 million doses.
“This new federal purchase can give Americans even more confidence that we will have enough supply to vaccinate every American who wants it by June 2021,” said Health and Human Services Secretary Alex Azar in a statement. The cost to taxpayers: $1.95 billion for the additional 100 million doses.
To aid vaccine production, the government said it is using its authority under a Cold War-era law that allows it to direct private manufacturing.
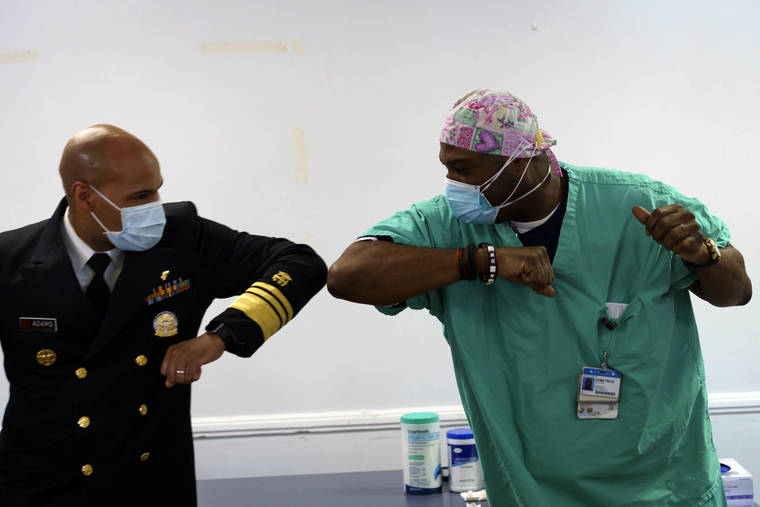
Pfizer’s vaccine was the first to be approved for emergency use by the Food and Drug Administration. It has now been joined by another two-shot vaccine from Moderna, developed in close collaboration with the National Institutes of Health. The government began shipping the Pfizer vaccine to states last week, and the one from Moderna this week.
The priority groups for first vaccination include health care workers and nursing home residents. Gradually more Americans will have access to the free vaccines, which have been shown to be highly effective in clinical studies undertaken so far.
Separately, HHS announced it has joined forces with another big pharma company — Merck— to support the large-scale manufacture of a promising treatment for patients suffering from severe COVID-19 illness.
The treatment, still under investigation and not yet approved by the FDA, is known as MK-7110. It has the potential to minimize the damaging effects of an overactive immune response to COVID-19. This immune overdrive unleashes a cascade of effects on the human body, complicating the life-saving efforts of doctors and nurses.
The government is paying Merck about $356 million to fast-track production of its treatment under the auspices of Operation Warp Speed, a joint effort between HHS, the Pentagon, and drug companies to develop vaccines and treatments for COVID-19. It’s the same collaboration that led to Moderna’s vaccine. The money will allow Merck to deliver up to 100,000 doses by June 30, if the FDA clears the treatment for emergency use.
The current wave of COVID-19 is straining hospitals in a number of states, from California to Pennsylvania, and Oklahoma to Rhode Island. Having better treatments would help keep patients out of intensive care, improving their chances of survival and reducing the burden and stress on hospital staff.
Under the Pfizer deal announced Wednesday, the company will deliver at least 70 million of the additional vaccine doses by June 30, with the remaining 30 million to be delivered no later than July 31.
“With these 100 million additional doses, the United States will be able to protect more individuals and hopefully end this devastating pandemic more quickly,” Pfizer CEO Albert Bourla said in a statement. “We look forward to continuing our work with the U.S. government and healthcare providers around the country.”
Pfizer initially had a contract through Operation Warp Speed to supply the government with 100 million doses of its vaccine. The drugmaker will receive nearly $2 billion for that deal as well.
The Associated Press previously reported that the government was close to reaching the just-announced deal with Pfizer in exchange for helping the company gain better access to manufacturing supplies.
A law dating back to the Korean War gives the government authority to direct private companies to produce critical goods in times of national emergency. Called the Defense Production Act, it’s expected to help Pfizer secure some raw materials needed for its vaccine.
“Operation Warp Speed and the Department of Health and Human Services are using the Defense Production Act to support the production of the six OWS-related vaccines, including Pfizer,” said HHS spokesman Michael Pratt. “Through the selective application of DPA authorities, OWS helps prioritize access to the critical materials and supplies necessary to expand vaccine production in support of U.S. government contracts.”
The other four vaccines are undergoing trials to determine their effectiveness and better understand their overall safety.
Pfizer and BioNTech undertook their own vaccine development, maintaining a more arms-length relationship with the government. But approval of their vaccine won them immediate name recognition, raising hopes of taming a pandemic that has killed more than 320,000 Americans and hobbled much of the economy. Local TV stations across the country began broadcasting scenes of doctors and nurses garbed in hospital scrubs receiving the first vaccinations. Some polls showed easing skepticism about getting vaccinated.
After early failures with testing, Trump administration officials are hoping to write a very different ending with vaccines. Operation Warp Speed has financed the development, manufacture and distribution of millions of doses, with the goal of providing a free vaccine to any American who wants one.
Operation Warp Speed is on track to have about 40 million doses of vaccine by the end of this month, of which about 20 million would be allocated for first vaccinations. Distribution of those doses would span into the first week of January. Both the Pfizer and Moderna vaccines require two shots to be fully effective.